Manufacturing Productivity and Polymer Performance Enhancements
January 30, 2003
Andre L. Lee, Ph.D., Michigan State University
Frank J. Feher, Ph.D., University of California, Irvine
Gerald F. Brem, Ph.D., California State University, Fullerton
John D. Barber, Hi-Tech Environmental Products, LLC
Abstract
The viscosity of polymers can be reduced at typical molding stresses and temperatures by the addition of a small amount (< 2 percent by weight) of a micron-scale solid. Although many solids may exhibit some effect on polymer viscosity, naturally occurring, non-toxic, amorphous aluminosilicate glass is the only solid that is effective over a wide range of polymer compositions and temperatures while at the same time having no demonstrable effect on nucleation of semi-crystalline polymers. Extensive research on poly(propylene), used as a representative polymer, indicates that rheologic properties described herein are dependent on particle composition, size range and concentration. The effect of the solid on the liquid polymer can thus be selected depending on the chosen particle characteristics. For most finishing applications, the practical benefit of the solid additive includes increased productivity, but may also include lower molding temperature, increased mechanical properties of the finished polymer, improved dispersion of additives, and better fit and finish of the article.
Introduction
Synthetic polymers are rapidly supplanting historically common raw materials such as metal, glass and wood. The continuing global transition to polymers as the raw materials of choice for many types of products will benefit industry and societies alike with these lightweight, but robust materials.
The expansion of polymers into applications traditionally utilizing other materials has resulted in an inherent conflict between different parts of the industry. Polymer manufacturers design and synthesize polymers to meet specifications and performance characteristics set by the end user. To meet increasingly higher performance objectives such as strength and toughness, polymer manufacturers are driven to manufacture products with higher molecular weights, narrower range of molecular weights, or complex polymer structures. The conflict arises when the finisher tries to produce finished articles from these polymers. The plastics finishers are challenged to produce articles with consistent quality and at production rates that are profitable. Typically, polymers with higher performance characteristics are more difficult to mold because higher performance is often associated with higher viscosity.
As if new, more difficult polymers were not enough, finishers are constantly challenged to reduce manufacturing costs even of traditional polymers, lest they lose the job to a competitor down the street or offshore.
Finishers have tried every imaginable technique to increase productivity, but all have substantial, if not unacceptable, drawbacks. Increased melt temperature, mechanical working of the polymer and/or injection/extrusion speed can increase the flow of viscous polymers, but a higher temperature generally increases the cycle time or results in degradation of the polymer or additives. Internal or external lubricants may improve melt flow, but their manufacture or use may have adverse environmental impacts, or their presence in the finished article may degrade mechanical properties of the articles or their ability to be painted or plated.
Fortunately, there has been a technological breakthrough that offers substantially improved polymer flow without any known adverse effects on polymer properties. Although it seems counterintuitive, the addition of a small amount of a solid to a polymer can result in a lower complex viscosity of the polymeric liquid at stresses and temperatures typical of the molding environment. A variety of solids can result in some amount of lower complex viscosity; however, naturally occurring aluminosilicate glass (trademarked name of Vitrolite®) is the only solid discovered to date that can simultaneously achieve significant viscosity reductions and the exacting demands of: (1) functionality in all polymeric compositions, (2) functionality over a range of polymer melt temperatures, (3) an increase in functionality with decreasing melt temperature, (4) no adverse effect on polymer integrity in excess of that associated with any polymer processing, (5) no change in nucleation or nucleation kinetics of semi-crystalline polymers, (6) no equipment degradation due to glass properties, (7) minimal or no change in mechanical properties of the polymer, (8) little or no change in finished polymer color, (9) increased dispersion performance of additives, and (10) complete medical and environmental safety.
The objective herein is to describe this breakthrough technology that is the subject of a pending patent. The emphasis will be on experimental results from use of an amorphous aluminosilicate glass in poly(propylene); however, we believe the fundamental explanations for the effects of glass in poly(propylene) apply also to other solids and other polymeric liquids. The effect of the solid on polymer properties can readily be demonstrated by several different analytical methods, but we will focus on dynamic methods that have proven to be highly effective and quantitative.
It is not our objective here to describe all of the potential benefits for the processor who adopts this technology, but suffice it to say that productivity increases of 15 to 40 percent are typical, reduction in processing temperature can result in improved mechanical properties of the polymer or less degradation of thermally sensitive polymers or additives, and additives can commonly be reduced without any decrease in performance.
Experimental Materials
Poly(propylene) used in this study was an injection grade, natural Pro-fax® 6524 from Basell. The poly(propylene) contains only low concentrations of thermal stabilizers.
The natural aluminosilicate glass was mechanically milled to a fine powder. Morphologic features of the particles were assessed by scanning electron microscopy (Figure 1).
Particle sizes and particle-size distributions were determined on a Beckman Coulter LS-230 laser diffractometer with Polarization Intensity Differential Scattering. Particle-size characterization from 0.04 to 2000 µm is possible with this instrumentation; however, particle sizes less than about 90 microns are typically most effective in altering polymer properties. The standard reference material used for these experiments was milled and classified to less than 800-mesh (less than ~20 µm by laser diffraction). A typical particle size distribution is shown in Figure 2. For some experiments, the 800-mesh glass was classified into a narrow size range of 3-11 and 6-16 µm.
The poly(propylene) pellets and glass powder were dried in a vacuum oven at 60oC overnight to remove moisture. Samples were compounded at 210oC for 20 minutes under a N2 atmosphere in a co-rotating Haake Mini-lab twinscrew extruder. The mixture was extruded and cut into pellets with a length of about 0.5 cm. The compounded PP/glass powder samples were pressed at 500 psi into 25-mm diameter by 1-mm thick discs in a 180oC hydraulic press. These discs were kept in a sealed desiccator prior to performing the dynamic viscosity experiments. Films of PP/glass powder for solid-property analysis were prepared under identical conditions.
Rheological Experiments
The rheological experiments were performed using a Paar Physica rheometer, Universal Dynamic Spectrometer UDS-200, equipped with a convection air oven for temperature control. Parallel-plate geometry with diameter of 25 mm and gap of 0.9 mm was used for all measurements obtained in this study. The fixture was preheated to the test temperature (180oC or 200oC), and then lowered to obtain the zero-gap reference point. The discshaped specimen was then placed in the rheometer; the fixture was slowly lowered to the recommended 0.9 mm gap with minimal normal force. Different specimens were used for tests at different temperatures.
Two types of rheological experiments were performed. The sample was first tested using a small-amplitude oscillatory shear with strain of 2% and oscillatory frequency from 100 to 0.1 radian/second. This type of experiment was used to compare the apparent complex viscosity at low oscillatory frequency as influenced by the addition of glass powders or other solid fillers. The sample was then tested at a fixed oscillatory frequency of 1 radian/second while the amplitude of shear stress was increased from 800 to 10,000 Pa. The experiment was terminated at less than the maximum shear stress if an edge fracture developed. Results from 10 Hz frequency shear-stress amplitude experiments will be reported elsewhere.
The solid mechanical properties were evaluated using a Rheometric Scientific, Inc. RAS-3 solid analyzer. The dynamic modulus, E*, was measured at temperatures ranging from 30 to 150oC with ramp rate of 2oC/minute at 6.26 radian/second and with an axial-strain amplitude of 0.05%.
Results
Intuitively, one would believe, and often be correct, that addition of a solid to a liquid increases the viscosity of the liquid. Polymeric liquids are similar to more common and simply behaved liquids – addition of a solid such as a filler, typically at concentrations of 5 weight percent or more, increases the viscosity and the viscosity increase correlates with increasing solid concentration. One might also believe that adding a low concentration of small solid particles to a polymeric liquid would have comparable effects on polymer viscosity; however, intensive study of polymeric melts with a low concentration of solid particles has revealed a far more complex behavior. All solids exhibit some of this complex behavior; however, natural aluminosilicate glass is the only known solid that exhibits a range of behaviors that can be of profound benefit to the polymer finishing industry.
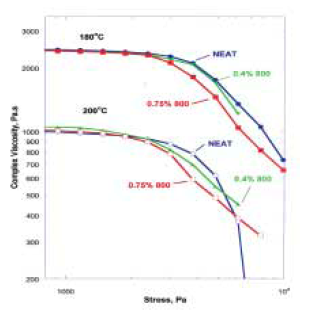
Under small-amplitude, oscillatory-shear conditions poly(propylene) (PP) with 0.75 weight percent 800-mesh glass particles has an apparent complex viscosity that is indistinguishable from NEAT PP (Figure 3).
This is not surprising considering the concentration of solid particles. Under higher amplitude shear-stress conditions, there are considerable differences between glass-containing PP and NEAT PP. Some of these differences, such as the higher apparent complex viscosity of glass-containing PP at shear-stresses below 2000 Pa (Figure 4),
are consistent with conventional expectations about the viscosity of mineral-filled thermoplastics. Other differences defy conventional expectations. In particular, the data from Figure 4 demonstrates clearly that there are circumstances where the complex viscosity of glass-containing PP is actually less than the viscosity of NEAT PP under identical shearstress conditions. This “window” of decreased viscosity for glass-containing PP, which is observed under shear-stress ranging from 2000 to 6200 Pa at 200oC, occurs because the shearstress dependence of complex viscosity is different for the two samples. In both cases, there is a dramatic (and expected) drop in complex viscosity beyond a critical shear-stress value, but this drop occurs at much lower shearstress value for glass-containing PP. At very high shear-stress >6200 Pa, the apparent complex viscosity of natural glass-containing PP is higher than NEAT PP. The reasons for this behavior are not known with certainty, but they are reproducible and are in accord with results from production-scale finishing of glass-bearing polymers.
The effect of glass particles on the apparent complex viscosity at high shear-stress amplitudes is dependent on solid concentration, temperature and particle-size range, although not in a linear manner.
At 200oC and with large shear-stress amplitude (Figure 5), an increase from 0.4 to 0.75 weight percent solid increases the viscosity reduction by up to 25 percent at shear stress >2000 Pa as might be expected. However, at lower shear stresses, the apparent viscosity of the PP with 0.4 weight percent glass particles is higher than that with 0.75 weight percent an unexpected result. The behavior is even more complex when the PP melt temperature is lowered to 180oC. A 0.4 weight percent concentration of glass particles has only a minimal effect on the apparent viscosity of the melt; however, a melt with 0.75 weight percent glass particles exhibits a very large viscosity reduction (38 percent) as compared to NEAT PP and a substantive decrease in the critical shearstress amplitude. They are somewhat inexplicable, but they are effects of solid concentration and melt temperature reproducible on dynamic analyzers. They are also broadly consistent with production-scale finishing experiments on a variety of polymer types where it can be demonstrated that there is an optimal solid concentration and that a lower melt temperature increases the deviation of the viscosity of glass-bearing polymeric melts from that of NEAT polymeric melts.
The size of glass particles has an orderly, albeit incompletely understood, effect on apparent viscosity. In small-amplitude oscillatory shear experiments, the apparent viscosity of glass-bearing PP decreases with particle size. That is, 325-mesh glass particles (<90 µm) are only about one-half as effective on an equal-weight basis as 800-mesh (<20 µm) particles. If the glass particles are classified into narrow size ranges, the apparent viscosity reduction becomes larger as the range decreases from 35-90 µm to 3-11 µm. With a further decrease in the particle size range, the trend reverses and the reduction in apparent viscosity becomes smaller with decreasing particle size. The effect of an optimal narrow particle-size range is substantial at low shearstress amplitudes (Figure 6) where the apparent complex viscosity of PP with glass particles is 40 percent less than that of NEAT PP. At high shear-stress amplitudes, glass particles classified into a 3-11 µm size range have the largest reduction in viscosity and greatest reduction in critical shear-stress amplitude of any size classification of glass particles.
It is difficult to succinctly or precisely describe at this time a mechanism whereby a low concentration of small solid particles, particularly of natural aluminosilicate composition, could lower the apparent complex viscosity. At low shear-stress amplitudes, is the reduction in viscosity due to particle-particle interaction, even though the volume fraction of particles is small? At high shear-stress amplitudes, there could be one or several mechanisms acting independently, additively or synergistically. One mechanism could be wall slip on the stationary bottom plate of the rheometer that is induced by drag flow. The addition of a small amount of solid glass particles is able to reduce the critical value of shear-stress amplitude, which induces drag flow under oscillatory shear. Alternatively, the reduced viscosity may be explained by interaction of solid particles with polymer chains. The non-linear viscoelastic response of polymers under large deformation may be related to higher stress concentration in molecular regions surrounding the natural glass particles. This in effect can lead to polymer chain alignment. These aligned chains move much easier as compared to un-oriented chains, which result in a significant reduction in the complex viscosity of the polymeric melt. The complex response of polymers with solids to temperature, particle concentration and particle sizes suggest that several mechanisms may be operative and their relative contributions are dependent on system properties and conditions.
It could be tempting to explain the effect of solid particles in PP or any polymeric melt to chemical reactions between the solid and polymer or to changes in polymer composition. Chemical reactions are all but ruled out because at least some of the effects attributed to solid particles are observed regardless of particle composition. We have concentrated on natural aluminosilicate glass because it appears to have the largest effect over the entire spectrum of polymer types and physicochemical conditions. Chemical decomposition of the polymer, chain scission or free radical reactions are also very unlikely because extensive testing has failed to detect any change in polymer integrity upon processing with glass particles.
If there is no discernable chemical reaction or alteration of the polymer integrity, then how does the presence of natural glass particles affect the physical/mechanical properties of the finished polymer? The presence of a low concentration of glass powder has only a minor effect on physical/mechanical properties of the PP, but even this minor effect can be mitigated. With exception of impact strength, all physical/mechanical properties of PP with glass are within the limits of experimental error as compared to NEAT PP. The impact strength of PP (as well as other polymers) with glass is lower by 10-15 percent, but this can be mitigated by processing at a lower melt temperature an option made possible by the reduction in melt viscosity due to glass particles. Additional improvements in mechanical properties such as flexural modulus occur as a result of a lower melt temperature in some polymers.
A small-amplitude, oscillatory-tensile experiment further demonstrates the effect of glass in PP and the effect of particle size. At a concentration of 0.75 weight percent, 800-mesh glass particles do not have any demonstrable effect on the dynamic tensile modulus (Figure 7).
On the other hand, addition of 0.75 weight percent glass particles classified to 6-16 µm, has a substantially higher E’ modulus, thus indicating a greater heat deflection temperature (Figure 8).
Conclusions
The addition of a low concentration of a solid (typically less than 2 percent, but often less than 1 percent) to a polymer has unanticipated and previously undocumented effects on the viscosity of polymeric melts. As exemplified by detailed investigation of poly(propylene) and less intensive investigation of a broad range of NEAT and filled semi-crystalline and amorphous polymers, the complex viscosity of a polymeric liquid is lower than the equivalent NEAT polymer under conditions typical of polymer finishing. The lower apparent viscosity was first demonstrated in a production-scale environment, but has subsequently been documented by dynamic-mechanical analytical methods.
Dynamic-mechanical analyses indicate that the complex viscosity of a polymeric liquid containing a solid can be lower under smallamplitude shear-stress conditions as well as large-amplitude shear-stress conditions. It is unclear how a low concentration of small particles, particularly of a narrow size distribution can lower the melt viscosity under low-amplitude shear stress. At high amplitude shear stress, the particles lower the critical stress amplitude, allowing for a stress regime of lower melt viscosity as compared to NEAT polymer. The overall mechanism is not fully understood, but most likely involves several different mechanisms that may be variably independent, cumulative or synergistic depending on particle concentration, particle size range, temperature and possibly shear stress.
The magnitude and direction of viscosity changes in the polymeric liquids are dependent on at least four factors. (1) The composition of the solid. All solids have some effect; however, natural aluminosilicate glass appears to be most effective in reducing polymer viscosity over a range of stresses and temperatures. (2) The size range of solid particles. Glass powder with a maximum size of 20 microns may require a concentration of only ~0.5 weight percent, whereas, powder with a maximum laserdiffraction size of ~90 microns may require about 1.0 weight percent to achieve a comparable viscosity reduction. Furthermore, powders classified to narrow size ranges within a range of 3-16 microns are even more effective in reducing polymer viscosity over a broader range of shear stresses. (3) The concentration of solid particles. If the concentration is too low, the viscosity reduction may be minimal. On the other hand, if it is too high, the complex viscosity of polymer with solid may be only slightly less than that of NEAT polymer. If particle concentrations are very high (e.g., 5-10 percent), their effect is analogous to those typical of solid fillers they increase polymer viscosity. (4) The melt temperature. The reduction in viscosity of a polymer with an appropriate solid load increases with decreasing temperature. Particle shape and surface morphologic features are believed to be a fifth determinant of how solid particles affect polymer viscosity; however, data are still too preliminary to draw definitive conclusions.
The most critical discovery, at least for the polymer finishing industry, is that use of a milled natural aluminosilicate glass with the correct particle-size range and concentration, will reduce the apparent viscosity of an extremely wide range of polymer types, both NEAT and filled. This facilitates finishing of polymers at much improved productivity rates. The melt temperature can often be reduced, particularly for polyolefins and nylons. If the melt temperature is reduced and frictional sources of heat are carefully managed, productivity increases of 15-40 percent can be achieved in injection and extrusion finishing. Furthermore, lower melt temperatures may result in enhanced mechanical properties of the finished polymer. Thermally unstable polymers or additives are less prone to decomposition under these lowertemperature conditions. And, as a final advantage for the polymer finisher, additives such as pigments, stabilizers, fire-retardant packages and impact modifiers can often be reduced with no decrease in the efficacy of the additive. Improved efficacy of additives is ascribed to the presence of natural glass particles. The particles increase polymer melt viscosity at very high shear-stress values, which in turn facilitates dispersion and distribution of companion additives.
Reproduced and published by: Society of Plastics Engineers – presented at ANTEC 2003 Executive Conference Management – presented at Additives 2003